Key Market Insights
- Over 175 players worldwide claim to have the necessary capabilities to offer fill / finish services for a variety of biological interventions
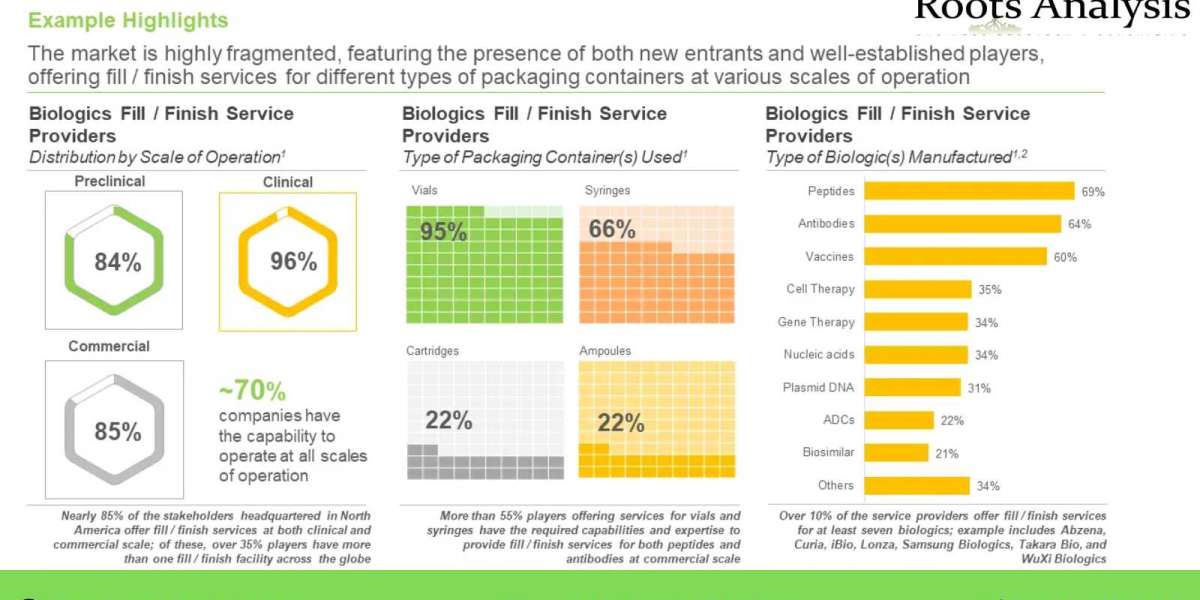
- The market is highly fragmented, featuring the presence of both new entrants and well-established players, offering fill finish services for different types of packaging containers at various scales of operation
- To cater to the evolving needs of clients / sponsors across the world, companies have established presence in multiple regions around the globe; Europe is the hub for fill / finish facilities
- In pursuit of building a competitive edge, stakeholders are actively upgrading their existing capabilities and enhancing their respective service offerings to comply with the evolving industry benchmarks
- Over the past few years, the field has witnessed a notable increase in expansion and partnership activity for biologics fill / finish capabilities
- Majority of the fill / finish capacity is installed in facilities established by large players; more than 40% of the available capacity is installed in facilities located in Europe
- By 2035, over 50% of the demand for fill / finish services is likely to be generated from filling of vials; a sizeable proportion of the current demand is being generated from commercialized drug products
- In order to meet the anticipated demand growth in the foreseen future, we expect stakeholders to continue to expand their facilities to further strengthen the supply-side
- The overall opportunity in biologics fill / finish services market is likely to grow at a CAGR of ~10%; it is expected to be well distributed across different scales of operation, types of biologics and packaging containers
- In the long-term, the majority share of revenues is likely to be driven by players offering fill/finish services for products intended for treating oncological and infectious diseases
Table of Content
- PREFACE
1.1. Scope of the Report
1.2. Market Segmentation
1.3. Research Methodology
1.4. Key Questions Answered
1.5. Chapter Outlines
- EXECUTIVE SUMMARY
- INTRODUCTION
3.1. Chapter Overview
3.2. Introduction to Biologics
3.3. Need for Outsourcing Biologics-related Operations
3.4. Introduction to Contract Manufacturing
3.5. Commonly Outsourced Operations in the Biopharmaceutical Industry
3.6. Basic Guidelines for Selecting a Fill / Finish Service Provider
3.7. Advantages of Outsourcing Fill / Finish Operations
3.8. Risks and Challenges of Outsourcing Fill / Finish Operations
- Biologics Fill / Finish Service providers: MARKET LANDSCAPE
4.1. Chapter Overview
4.2. Biologics Fill / Finish Service Providers: Overall Market Landscape
- Company Competitiveness Analysis
5.1. Chapter Overview
5.2. Methodology and Key Parameters
5.3. Biologics Fill / Finish Service Providers in North America
5.4. Biologics Fill / Finish Service Providers in Europe
5.5. Biologics Fill / Finish Service Providers in Asia-Pacific and Rest of the World
- BIOLOGICS FILL / FINISH SERVICE PROVIDERS IN NORTH AMERICA
6.1. Chapter Overview
6.2. AbbVie Contract Manufacturing
6.2.1. Company Overview
6.2.2. Service Portfolio
6.2.3. Financial Information
6.2.4. Recent Developments and Future Outlook
6.3. BioPharma Solutions
6.4. BioReliance
6.5. Catalent Biologics
6.6. Patheon
6.7. Charles River Laboratories
- Biologics Fill / Finish Service Providers in Europe
7.1. Chapter Overview
7.2. Boehringer Ingelheim BioXcellence
7.2.1. Company Overview
7.2.2. Service Portfolio
7.2.3. Financial Information
7.2.4. Recent Developments and Future Outlook
7.3. Lonza
7.4. Pierre Fabre
7.5. Recipharm
7.6. Wacker Biotech
7.7. Fareva
7.8. Fresenius Kabi
7.9. Glaxo SmithKline
- Biologics Fill / Finish Service Providers in Asia-Pacific
8.1. Chapter Overview
8.2. Asymchem
8.3. Samsung Biologics
8.4. Syngene
8.4.1. Company Overview
8.5. Takara Bio
8.6. WuXi Biologics
8.7. Hetero Drugs
8.8. Intas Pharmaceuticals
- Partnerships and Collaborations
9.1. Chapter Overview
9.2. Partnership Models
9.3. Biologics Fill / Finish Service Providers: List of Partnerships and Collaborations
- Recent Expansions
10.1. Chapter Overview
10.2. Biologics Fill / Finish Service Providers: Recent Expansions
- Capacity Analysis
11.1. Chapter Overview
11.2. Assumptions and Methodology
11.3. Global Fill / Finish Capacity (by Number of Units Filled)
11.4. Global Fill / Finish Capacity (by Fill Volume)
11.5. Global Fill / Finish Capacity for Ampoules (by Number of Units Filled)
11.6. Global Fill / Finish Capacity for Ampoules (by Fill Volume)
11.7. Global Fill / Finish Capacity for Cartridges (by Number of Units Filled)
11.8. Global Fill / Finish Capacity for Cartridges (by Fill Volume)
11.9. Global Fill / Finish Capacity for Syringes (by Number of Units Filled)
11.10. Global Fill / Finish Capacity for Syringes (by Fill Volume)
11.11. Global Fill / Finish Capacity for Vials (by Number of Units Filled)
11.12. Global Fill / Finish Capacity for Vials (by Fill Volume)
11.13. Conclusion
- Demand Analysis
12.1. Chapter Overview
12.2. Assumptions and Methodology
12.3. Global Demand for Biologics Fill / Finish Services
12.4. Demand and Supply Analysis
- KEY PERFORMANCE IndicaToRs FOR BIOLOGIC MANUFACTURING AND FILL / FINISH
13.1. Chapter Overview
13.2. Biologics Manufacturing and Fill / Finish: Key Performance Indicators
- regional Capability AssessMent
14.1. Chapter Overview
14.2. Assumptions and Key Parameters
14.3. Biologics Fill / Finish Capabilities in North America
14.4. Biologics Fill / Finish Capabilities in Europe
14.5. Biologics Fill / Finish Capabilities in Asia-Pacific and Rest of the World
14.6. Regional analysis of installed biologics fill / finish capacity
14.7. Concluding Remarks
- Market Sizing and Opportunity Analysis
15.1. Chapter Overview
15.2. Forecast Methodology
15.3. Overall Biologics Fill / Finish Services Market, 2022-2035
15.4. Biologics Fill / Finish Services Market for Ampoules, 2022-2035
15.5. Biologics Fill / Finish Services Market for Cartridges, 2022-2035
15.6. Biologics Fill / Finish Services Market for Syringes, 2022-2035
15.7. Biologics Fill / Finish Services Market for Vials, 2022-2035
- Future Growth Opportunities
16.1. Chapter Overview
16.2. Growing Biopharmaceutical Pipeline
16.3. Increase in Outsourcing of Fill / Finish Activities
16.4. Rising Focus on Self-Administration of Drugs
16.5. Advances in Aseptic Fill / Finish Technologies
16.6. Growing Opportunities in the Asia-Pacific Region
- Case Study: Robotic Systems in Fill / Finish Operations
17.1. Chapter Overview
17.2. Contract Service Providers: List of Fill / Finish Equipment
17.3. Role of Robotic Systems in Fill / Finish Operations
17.4. Companies Providing Robots for Use in the Pharmaceutical Industry
17.5. Companies Providing Isolator-based Aseptic Filling Systems
17.6. Concluding Remarks
- Case Study: Ready-to-Use Packaging Components for Aseptic Fill / Finish
18.1. Chapter Overview
18.2. Role of Ready-to-Use Packaging Components in Aseptic Fill / Finish Operations
18.3. Companies Providing Ready-to-Use Packaging Components
18.4. Conclusion
- Concluding Remarks
- Executive Insights
20.1 Chapter Overview
20.2 IDT Biologika
20.3 Cytovance Biologics
20.4. Syngene
20.5 oncomed manufacturing
20.6 Yposkesi
20.7 HALIX
- Appendix 1: Tabulated Data
- Appendix 2: List of Companies and Organizations
To view more details on this report, click on the link
https://www.rootsanalysis.com/reports/view_document/biologics-fill-finish-services-market/256.html
You may also be interested in the following titles:
About Roots Analysis
Roots Analysis is a global leader in the pharma / biotech market research. Having worked with over 750 clients worldwide, including Fortune 500 companies, start-ups, academia, venture capitalists and strategic investors for more than a decade, we offer a highly analytical / data-driven perspective to a network of over 450,000 senior industry stakeholders looking for credible market insights.
Contact:
Ben Johnson
+1 (415) 800 3415